 Introduction
Introduction
Jimma University (JU) is a public higher educational institution established in December 1999 by the amalgamation of Jimma College of Agriculture (founded in 1952), and Jimma Institute of Health Sciences (established in 1983).
JU is engaged in research, training and community services. It is laying the ground work for the development of pharmaceutical industry through conducting pharmaceutical research and training of pharmaceutical professionals in the country.
Jimma University Laboratory of Drug Quality (JuLaDQ) was established in 2009/10 as part of IUC-JU collaborative research program between Jimma University and consortium of Flemish Universities (Drug Quality and Registration (DruQuaR) of Ghent University), Belgium and became operational since 2011. JuLaDQ has a well-established quality system based on quality management guidelines of WHO and ISO/IEC 17025 where the processes of prequalification and accreditation are on progress. In 2016 GC, the name JuLaDQ is changed into Drug Quality Research Center. Currently, Drug Quality Research Center has fit-for-purpose qualified personnel and facility comprising ultrapure water purification system, High Pressure Liquid Chromatography (HPLC), dissolution apparatus, UV-Visible Spectrophotometer, etc. and serving as the center for research and training. The research particular focuses on medicines used for neglected tropical diseases, malaria, tuberculosis and HIV/AIDS as well as discovery and development of new drugs for infectious diseases.
Vision
- To be recognized as a global leader in pharmaceutical analytical research, training and services.
Mission
- To actively involve in research and training and service to support regulatory compliance with new and existing medicines.
Strategic objectives
- To support human resource development in pharmaceutical sciences and beyond.
- To support efficient and effective pharmaceutical regulatory systems in the country and beyond.
- To provide a GLP/GMP compliant accredited pharmaceutical analytical facility for research, training and service in pharmaceutical quality assurance and control and other related regulatory activities.
- To provide an independent laboratory for analysis and quality control of human and veterinary pharmaceutical, biotechnological, and complementary medicines.
- To provide the required support to pre-clinical and clinical development of potential new medicines and drug delivery systems
Organogram
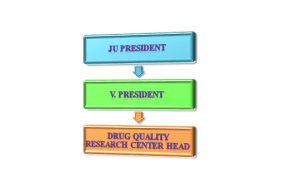
DQRC/JuLaDQ organogram

Researchers conducting their research at Drug Research Center

HPLC equipment at Drug Research Center and documentation system

Professor Sultan Suleman establisher of JuLaDQ
 After a symposium on Quality pharmaceutical Drugs: from order to patient.
After a symposium on Quality pharmaceutical Drugs: from order to patient.
JU Laboratory of Drug Quality |
